Featured
Stage 3 Clinical Trials
Phase III of a clinical trial usually involves up to 3000 participants who have the condition that the new medication is meant to treat. 20 to 100 healthy volunteers or people with the diseasecondition.
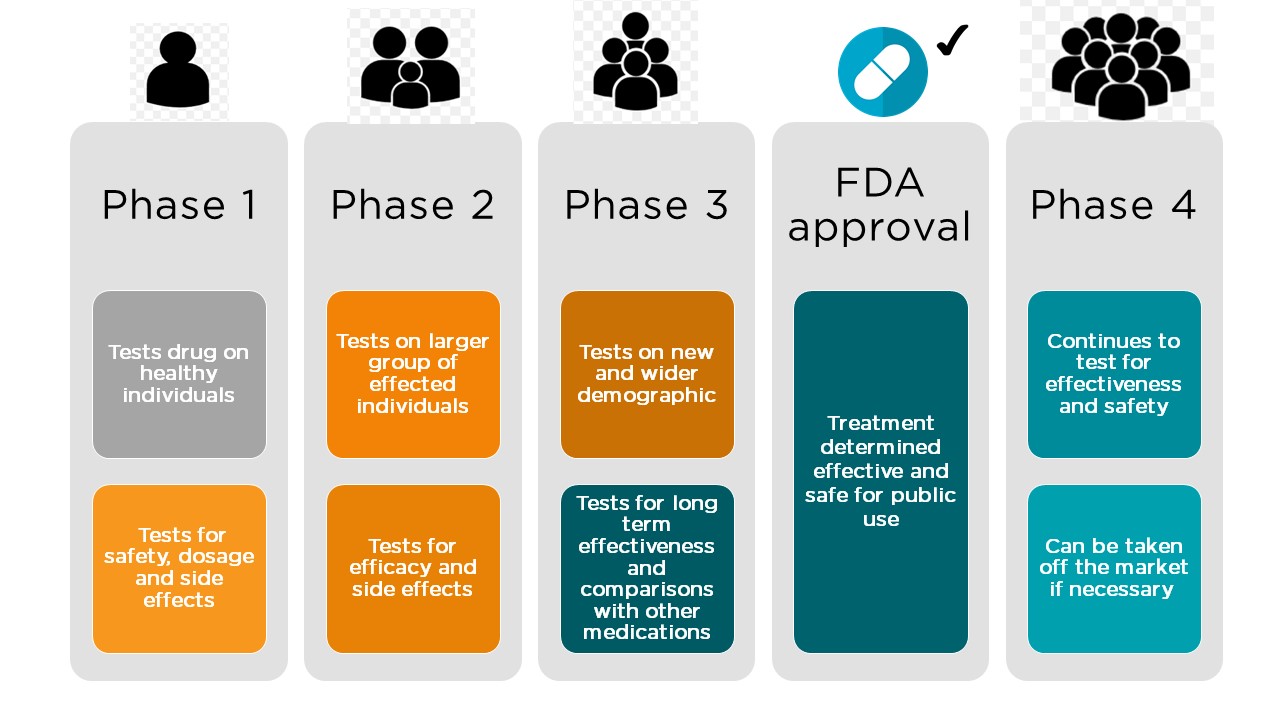 Clinical Trial Faqs Nephcure Kidney International
Clinical Trial Faqs Nephcure Kidney International
This also includes patients with a history of lower stage melanoma and subsequent recurrent metastatic disease that is either locallyregionally advancedinoperable disease or distant metastases.
Stage 3 clinical trials. When phase 3 clinical trials or sometimes phase 2 trials show a new drug is more effective and safer than the current standard treatment a New Drug Application NDA is submitted to the Food and Drug Administration FDA for approval. The most reliable study design is a randomized blinded and placebo- or active-controlled trial to verify that the results are beneficial. On July 28 2017 MAPS and the FDA reached agreement on the Special Protocol Assessment for Phase 3 clinical trials.
Researchers design clinical trials to answer specific research questions related to a medical. Many clinical trials look at new ways to detect diagnose or measure the extent of disease. Citius Pharmaceuticals Reports Strong Clinical Community Engagement During Mino-Lok Phase 3 Trial-Related Webinar.
NYSEPFE today announced the initiation of four Phase 3 clinical trials within its current pipeline of investigational. Clinical Research Phase Studies. A Phase 3 Clinical Trial involves a much larger group of volunteers and primarily focuses on determining whether the treatment would be safe and effective for a wide variety of people.
The final clinical trial stage is Phase 4 or the post marketing surveillance phase. And a respiratory syncytial virus vaccine candidate in pregnant women Pfizer Inc. Clinical trials can vary in size and cost and they can involve a single research center or multiple centers in one country or in multiple countries.
Clinical Research Designing Clinical Trials. Measurable disease according to RECIST version 11. The Investigational New Drug.
Phase 3 clinical trials are designed to test whether the investigative treatment is better than the standard clinical method for the targeted health condition. Researchers still use human volunteers to test these methods and the same rules apply. A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun.
Food and Drug Administration FDA. A pentavalent meningococcal vaccine candidate in adolescents. A Phase 3 clinical trial lasts one to four years and only about 25 to 35 percent of the drugs move onto the final phase.
After a successful Phase 3 trial vaccine manufacturers submit an application to regulatory bodies such as the European Commission or the US. Food and Drug Administration considers approving it. Costs for clinical trials can.
Phase III trials are usually large some involve tens of thousands of participants and are done at many places in the United States and sometimes around the world. At this stage clinical trial data is reviewed to make sure the vaccine is safe and effective. Phase 3 trial to evaluate efficacy and safety of AT-527 an oral antiviral for treatment of patients with mild to moderate COVID-19 in outpatient setting.
A phase III trial is the last step a new treatment goes through before the US. Clinical trials are studies to test new drugs already approved drugs devices or other forms of treatments. The NDA which includes data from all the pre-clinical and clinical studies is reviewed by the FDA.
Some even look at ways to prevent diseases from happening. Phase 3 is the final phase of clinical trials for an experimental new drug embarked upon if Phase 2 trials show evidence of effectiveness. Clinical study design aims to ensure the scientific validity and reproducibility of the results.
First subjects recently administered immunizations in two studies of 20-valent pneumococcal conjugate vaccine candidate in infants. Usually two or more phase 3 trials are conducted. The vaccine known as mRNA-1273 was co-developed by the Cambridge Massachusetts-based biotechnology company Moderna Inc and the National Institute of Allergy and Infectious Diseases NIAID part of the National Institutes of Health.
This includes American Joint Committee on Cancer AJCC stage IV or advancedinoperable stage III. The plan normally involves assigning participants to treatment or control groups. Trials in this phase can last for several years.
This agreement confirms that the protocol design clinical endpoints planned conduct and statistical analyses for the Phase 3 trials MAPP1 and MAPP2 are acceptable to support regulatory approval by the FDA. These trials enroll the most individuals usually several thousand.
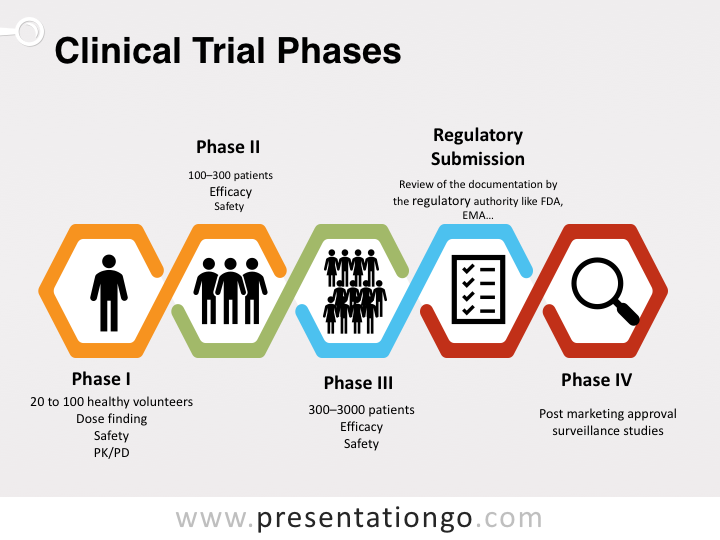 Clinical Research Basics Clinical Trials Are Part Of The New By Clinical Research Hub Medium
Clinical Research Basics Clinical Trials Are Part Of The New By Clinical Research Hub Medium
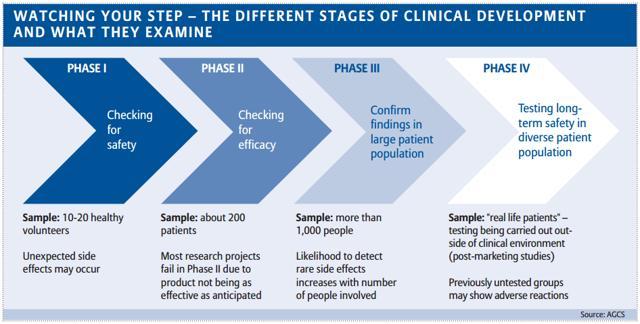 Investor S Guide To Clinical Trials Phase Success Rates For Introductory Pipeline Analysis Seeking Alpha
Investor S Guide To Clinical Trials Phase Success Rates For Introductory Pipeline Analysis Seeking Alpha
Phases Of A Trial Treatment Lupus Clinical Trials
 Phases Of Clinical Research Wikipedia
Phases Of Clinical Research Wikipedia
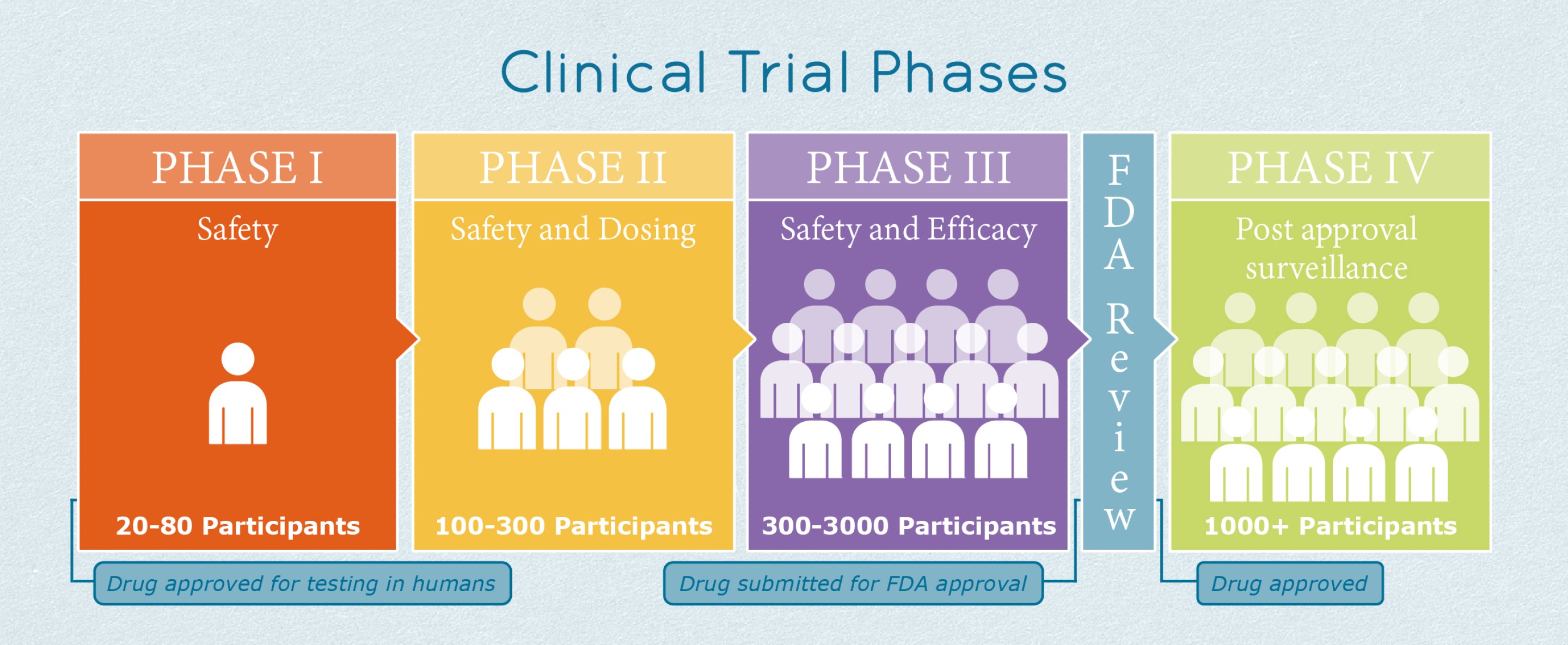 Phase Iii Ipf Clinical Trials Ild Collaborative
Phase Iii Ipf Clinical Trials Ild Collaborative
 Clinical Trial Phases Ifopa International Fibrodysplasia Ossificans Progressiva Association
Clinical Trial Phases Ifopa International Fibrodysplasia Ossificans Progressiva Association
 Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals
Understanding Clinical Trial Terminology What S A Phase 1 2 Or 3 Clinical Trial Concert Pharmaceuticals
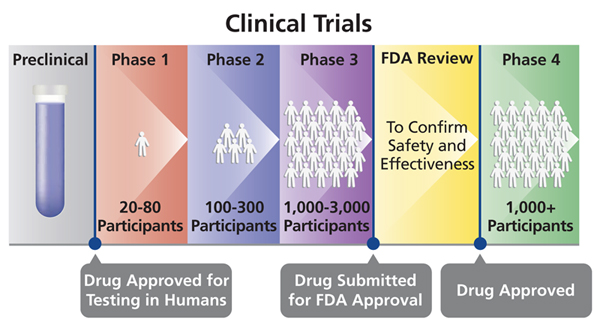 Different Phases Of Clinical Trials Drugs Testing And Development Of Vaccines In Clinical Research Online Science Notes
Different Phases Of Clinical Trials Drugs Testing And Development Of Vaccines In Clinical Research Online Science Notes
Phase Iii Trial Failures Costly But Preventable
 How Do Clinical Trials Progress Cancer Institute Nsw
How Do Clinical Trials Progress Cancer Institute Nsw
On Biostatistics And Clinical Trials
Popular Posts
I Think I Need To Go To A Mental Hospital
- Get link
- X
- Other Apps



Comments
Post a Comment